1.3: Factors Affecting Reaction Rate
The sentences with red color are the main point in explaining factors of concentration, pressure, particle size & temperature affecting reaction rate.
***For temperature & catalyst, they are related with the activation energy
FACTOR - Temperature
If the explanation about general effect of Temperature to the rate of reaction, we use the explanation given above.
If specifically explanation about effect of 2 different Temperature to the rate of reaction, we must refer to MAXWELL - BOLTZMANN DISTRIBUTION CURVE
Diagram 1: Energy profile of exothermic & endothermic (with & without catalyst)
💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨
CALCULATION USING ARRHENIUS EQUATION
Temperature & Activation energy with presence of catalyst are involved in Arrhenius equation. Thus, we must know the effect when change on Temperature & Activation Energy occur.
👉
💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨
***R = gas constant (8.314 J/mol K)
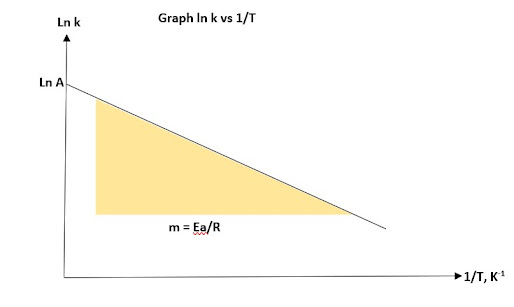
Arrhenius equation also can be used as graphical method like integrated rate law in Chapter 1.1: Reaction Rate.
But we use the Arrhenius equation for graphical method using
ln k = -Ea/R (1/T) + ln A
*** it must obey y = mx + c method in plotting the graph
Therefore, Graph ln k against 1/T will be plotted
💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨💨
Arrhenius Equation using for calculation
Normally, this equation is used when we have 2 different temperature with different rate constant, k.










No comments:
Post a Comment