1.2: COLLISION THEORY
COLLLISION THEORY ONLY OCCUR WHEN:-
Chart 1: Criteria of Collision Theory
In CHEMISTRY, focus on EFFECTIVE COLLISION.
Chemical reaction that produce PRODUCT very important & occur because of EFFECTIVE COLLISION
One of the criteria to form EFFECTIVE COLLISION is colliding molecules possess a minimum energy (ACTIVATION ENERGY)
WHAT IS ACTIVATION ENERGY?
Ø Activation energy (Ea), is the minimum
energy required for effective collisions in order to initiate
a chemical reaction
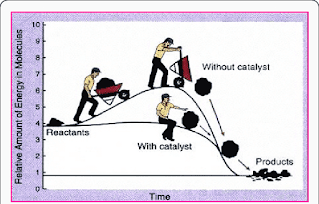
Diagram 2: Analogy of Activation energy
Normally, chemical reaction without catalyst has higher peak for activation energy. Thus, the rate of reaction become very slow.
Chemical reaction with catalyst has lower peak for the activation energy. Thus, the rate of reaction become very fast.
The peak of activation energy can be seen in the ENERGY PROFILE DIAGRAM. There are 2 type of chemical reactions (EXOTHERMIC & ENDOTHERMIC) that can represent by energy profile diagram.
Table 1: Comparison of Energy Profile for Exothermic & Endothermic Reaction
TRANSITION STATE is occurred in the area of ACTIVATION ENERGY with MAXIMUM POTENTIAL ENERGY.
During the chemical reaction, the collision is happened & if the effective collision form, ACTIVATED COMPLEX (TEMPORARY SPECIES) will produce at the stage we called TRANSITION STATE






No comments:
Post a Comment